Clinical trials
Our service covers phase I to phase IV clinical study of drugs. Our technicians have many years of experience in international multi-center clinical trial registration as well as rich research experience in over 20 fields including respiration, digestion, oncology, internal secretion, neurology, and nutriology. We have an efficient and professional CRA team distributed in over 20 provinces and municipalities in China to provide convenient and high-quality project management and monitoring service for you.
- Project management: In terms of project management, ERD is capable of formulating and implementing project management plan, risk management plan, and budget management plan to assist clients to complete site screening, investigator selection and patient enrollment at the fastest pace.
- Clinical monitoring: During clinical monitoring service, ERD strictly follows our clients’ SOPs or our own SOPs in all works related to the monitoring such as verification of case report form and original data and resolution of data query. And we archive data in a reasonably way.
- Clinical trial audit: We provide clinical trial audit service including drug clinical trial audit and medical device clinical trial audit, and can carry out audit on study site as well as sponsor’s study documents. All our auditors have more than 10 years of experience in clinical trial. Since the establishment of our company, we have carried out clinical audit for projects including international multi-center clinical trial and registered clinical trial. We have also accumulated extensive experience in third-party audit for self-verification projects with regard to drugs and medical devices. The standards we adopt for audit include ICH GCP, CFDA GCP, and relevant laws and regulations of China, and our clients are mostly well-known global and domestic pharmaceutical enterprises.
- Medical writting: Our medical writing team can provide professional medical writing service, which includes I-IV phase and BE study protocol, medical records, case report form, informed consent form, investigator's brochure, clinical trial report, clinical evaluation report, clinical evaluation risk assessment, clinical trial review and medical review according to NMPA and ICH regulations.
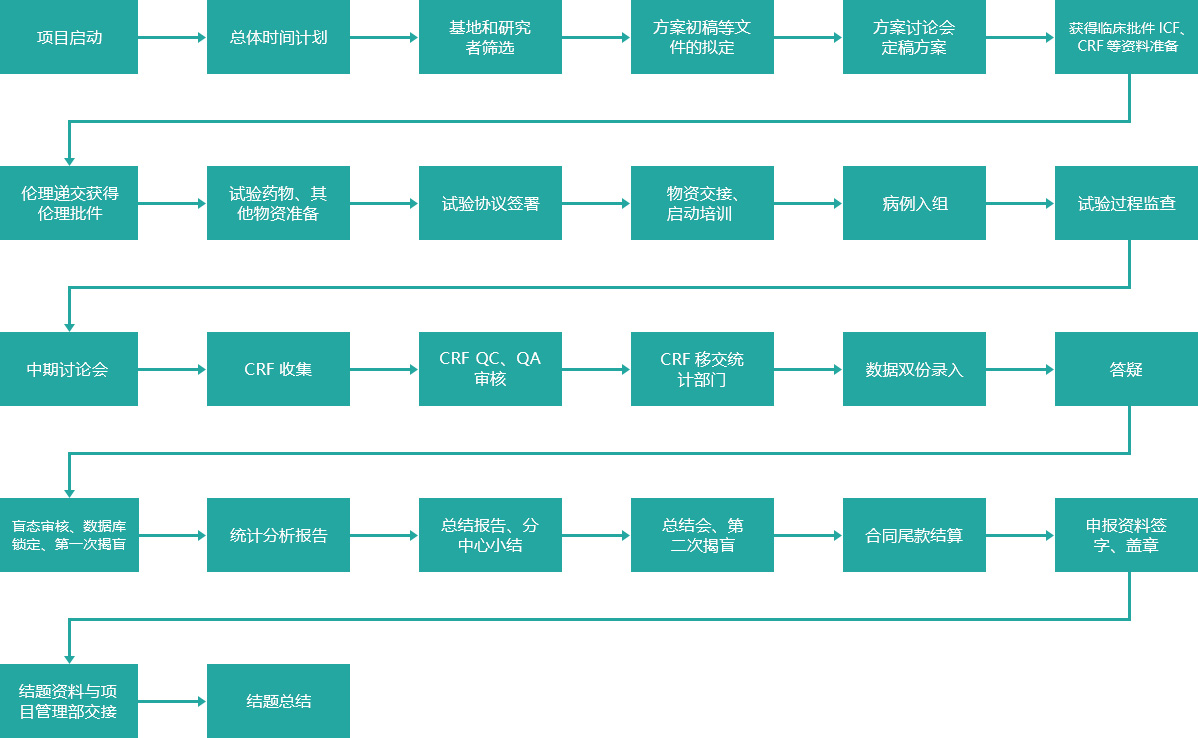
Schematic diagram of clinical trial process